Intrinsically disordered regions (IDRs) and low complexity domains (LCDs), which lack predictable three-dimensional structures, are widespread features of the human proteome. These unstructured protein domains facilitate dynamic yet specific interactions that establish functional orders of cellular activities. Additionally, they are closely associated with various human diseases, including cancers, neurodegenerative disorders, and viral infections. However, the molecular mechanisms that govern their physiological functions and their transition to disease-associated states remain enigmatic. Our objective is to gain a deeper understanding of the roles played by disordered protein IDR/LCDs within cellular processes.
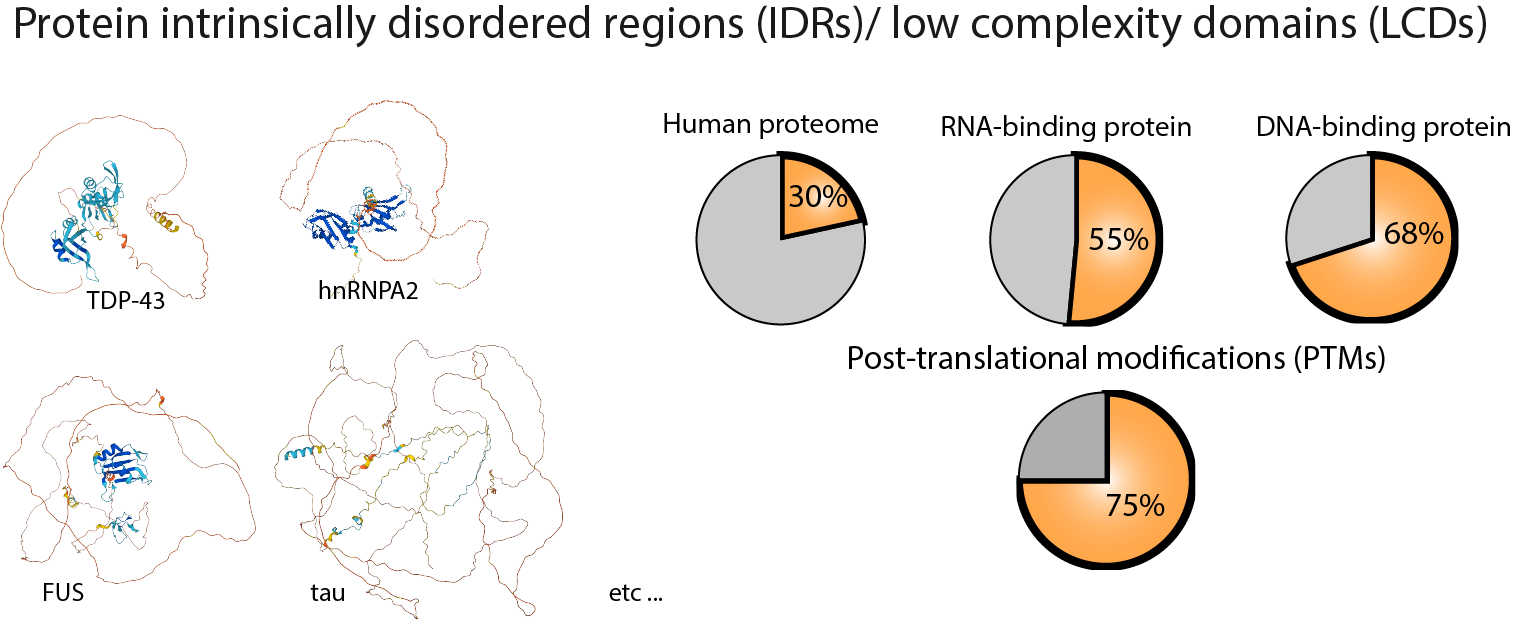
1. The role of IDR/LCDs in physiology
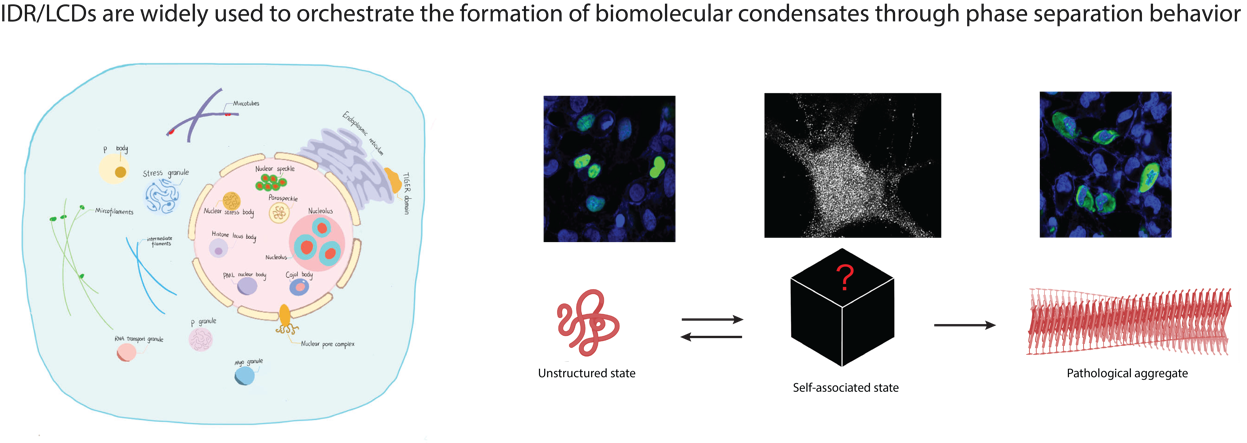
(1) Understanding How IDR/LCDs form biological condensates via liquid-liquid phase separation (LLPS).
In addition to organelles bound by lipid membranes, membraneless organelles and other biomolecular condensates have been widely discovered as novel mechanisms for compartmentalizing biological activities within cells with precision and efficiency. Protein regions known as IDR/LCDs are key components involved in the formation of these functionally specific subcellular condensates through a process called liquid-liquid phase separation (LLPS). However, the specific molecular mechanisms underlying the interactions mediated by protein IDR/LCDs remain to be elucidated. Our goal is to comprehensively understand the role of IDR/LCDs in the formation of phase-separated condensates within cells, as well as their contributions to diseases such as neurodegenerative diseases and cancers.
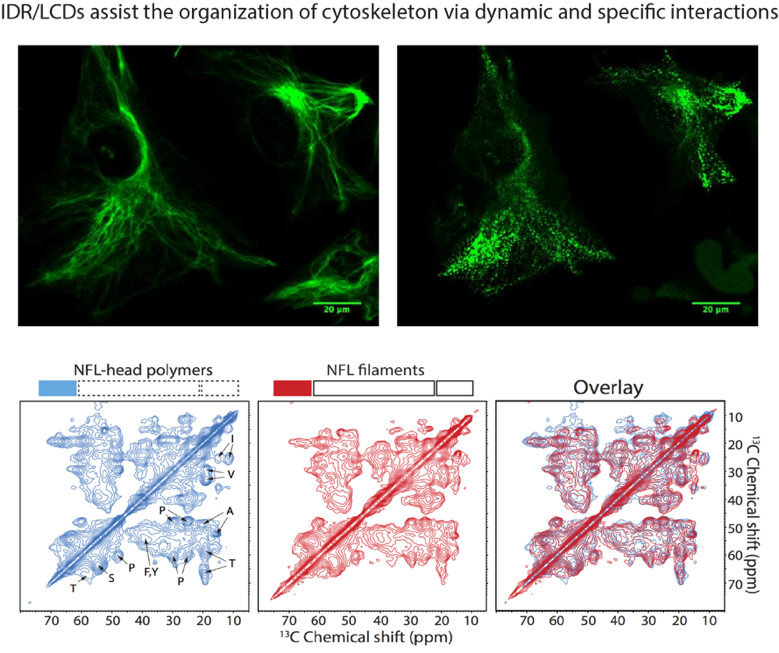
(2) How IDR/LCDs regulate cytoskeleton organization in cells.
IDR/LCDs in proteins such as tau, intermediate filament proteins, and formins play critical roles in the proper organization of the cytoskeleton, including microtubules, intermediate filaments, and actin filaments. Post-translational modifications within these IDR/LCDs are employed to regulate the cytoskeleton in response to functional demands. Aberrant behavior of IDR/LCDs can lead to human diseases and/or drug resistance by altering dynamic yet specific interaction patterns. Our goal is to understand how IDR/LCDs function spatiotemporally in the regulation of cytoskeletons in both physiological and pathological contexts.
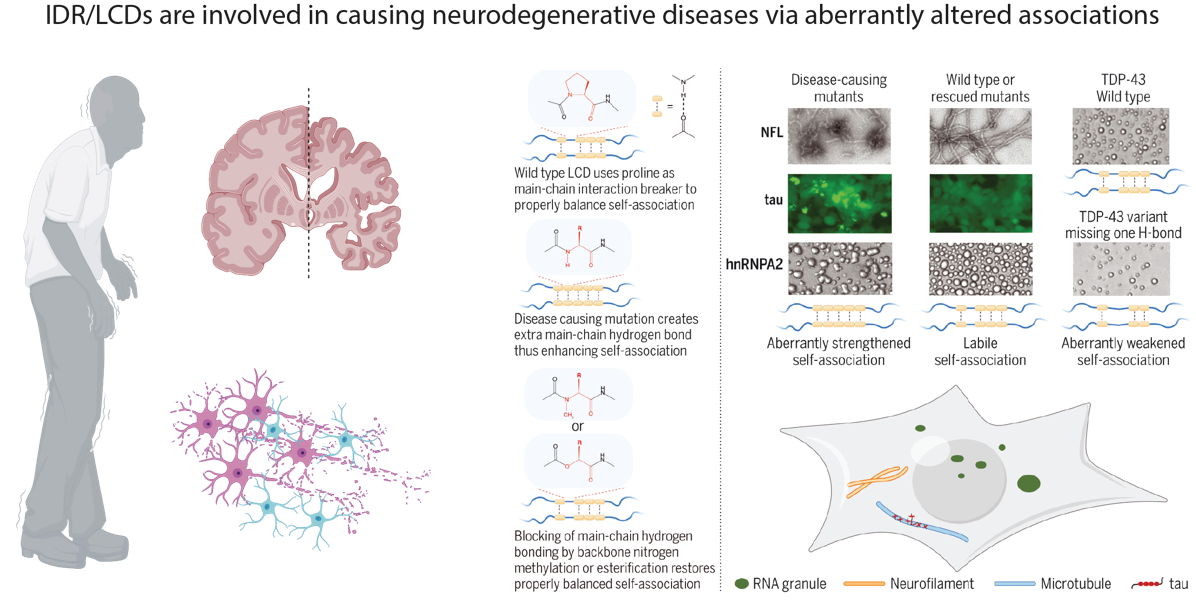
2. IDR/LCDs in aging and the related diseases
(1) The role of IDR/LCDs in causing neurodegenerative diseases
Neurodegenerative disorders encompass a broad spectrum of conditions caused by the progressive damage to cells and connections in the nervous system that are crucial for mobility, coordination, strength, sensation, and cognition. These diseases affect millions of people globally, and effective cures are still lacking for most of these intricate neurological conditions. Dysfunctions and abnormal aggregations of protein IDR/LCDs have been associated with the onset and progression of neurodegenerative disorders such as ALS, AD, PD, CMT, among others. To enhance our understanding of these devastating diseases related to aging and degeneration, and to inform the development of clinical strategies, we will delve into the molecular mechanisms that underlie the alterations in IDR/LCD-mediated interactions and functions leading to neurodegeneration.
![]()
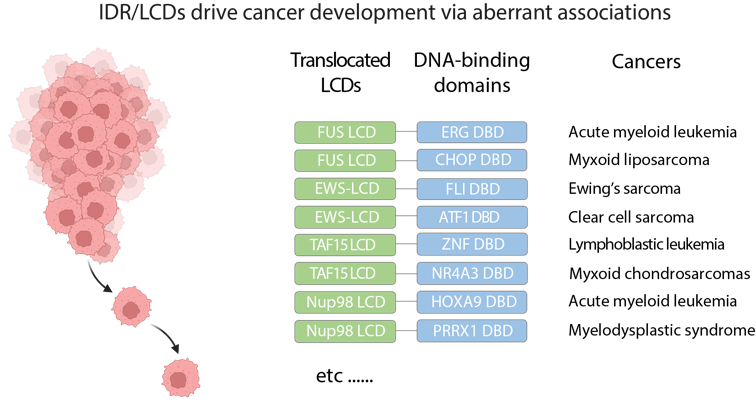
(2) The role of IDR/LCDs in cancer development
Aberrant intermolecular associations, such as phase separation mediated by IDR/LCDs, along with dysregulated expression of IDR/LCDs involved in gene regulation processes like transcription and splicing, contribute to the development of various types of cancers. Our research focuses on understanding how these unstructured IDR/LCDs promote cancer through altered phase separation behaviors and aims to identify new therapeutic targets or sites for potential treatments.
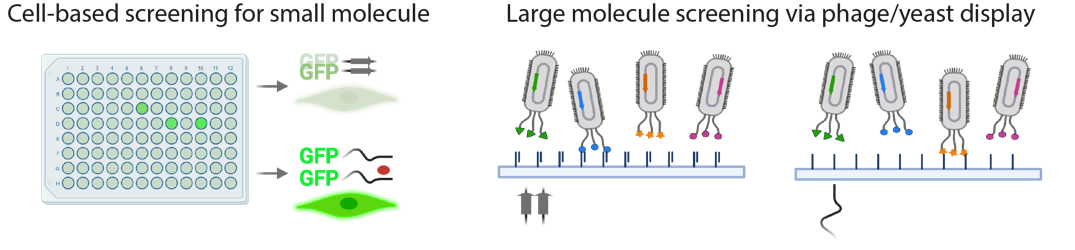
3) Strategies for manipulating IDR/LCD-mediated interactions in laboratory and clinical settings
Understanding the molecular basis of interactions mediated by IDR/LCD has opened new opportunities to target these disordered and traditionally 'undruggable' proteins. For example, by determining the transient structures of functionally important local regions within IDR/LCDs, we were able to inhibit the aberrant self-association or phase separation of neurodegeneration-related IDR/LCDs by blocking a single hydrogen bond. We are now developing high-throughput assays and conducting both cell-based and in vitro screenings of drug molecules.
![]()
![]()
![]()

